C–H Amination of Arenes and Heteroarenes through a Dearomative (3 + 2) Cycloaddition
Sajan Pradhan, Fahimeh Mohammadi, Rikuou Tanase, Kazuma Amaike, Kenichiro Itami*, Jean Bouffard*
J. Am. Chem. Soc. 2025, 147, 27731–27742.
DOI: 10.1021/jacs.5c06390
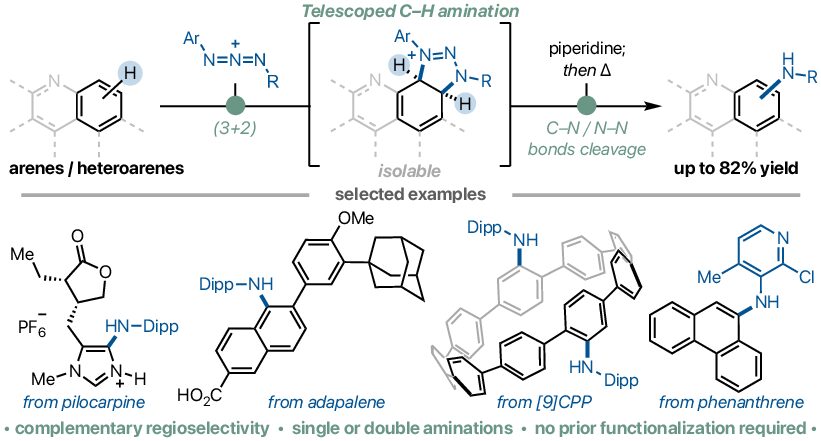
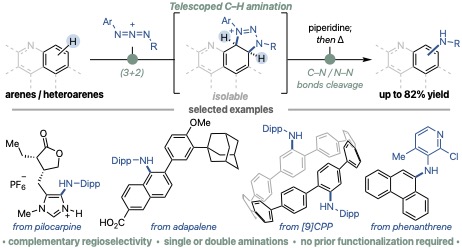
C–H amination of aromatic compounds is an attractive method for synthesizing important arylamines without the need for prior functionalization such as halogenation or metalation. Until now, these reactions have most often relied on transition-metal or photoredox catalysis, proceeding through mechanisms such as electrophilic substitution, radical addition, or nitrene insertion.
Through an international collaboration with the Jean Bouffard laboratory at Ewha Womans University (Korea), we developed a new method for C–H amination that can also be applied to aromatic and heteroaromatic compounds that have been difficult to functionalize. The reaction begins with a cycloaddition between an azidium ion and an aromatic substrate to generate an adduct, which is then transformed into diverse arylamines through mild base treatment followed by heating at moderate temperatures. This method exhibits a broad substrate scope and was demonstrated to be applicable to the synthesis of complex molecules such as natural products, pharmaceuticals, agrochemicals, and functional organic materials.
Furthermore, when compared to representative arylamine synthesis methods, such as halogenation followed by Buchwald–Hartwig amination, or Ir-catalyzed borylation followed by Chan–Lam coupling, our approach shows a complementary and unique regioselectivity. Notably, the achievement of selective double C–H amination of molecular nanocarbons such as coronene and [9]cycloparaphenylene represents a significant step toward the material and biological applications of molecular nanocarbons.


 日本語
日本語