A science that creates molecules and values
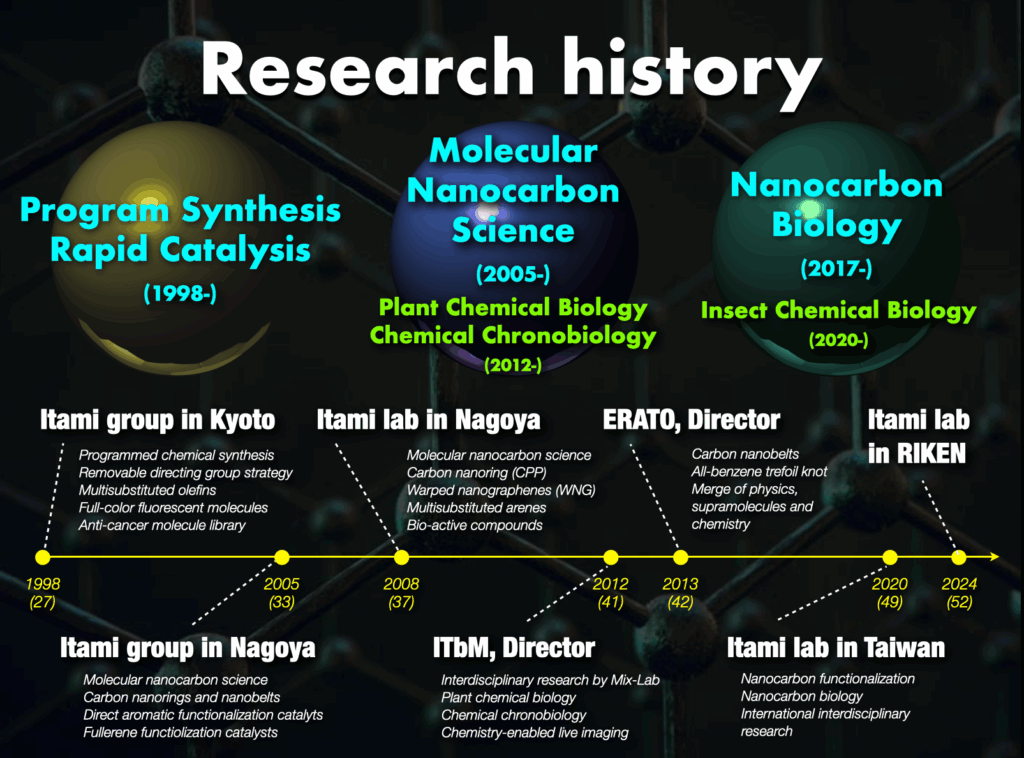
1. History of our research
The impact of molecules on science, technology, and society is profound, offering solutions to numerous challenges our planet faces. Our vision is to develop and introduce molecules with revolutionary functions or aesthetically pleasing structures to address these challenges. To date, we have successfully developed 23 molecules, including molecular nanocarbons, reagents, catalysts, ligands, and bioactive molecules, which are widely used by researchers globally.
My academic journey includes studies in organic chemistry, bio-related chemistry, organometallic chemistry, catalytic chemistry, and synthetic chemistry at Kyoto University (Japan) and Uppsala University (Sweden). These studies laid the foundation for subsequent research focused on creating new materials through molecular and synthetic chemistry. As an assistant professor at Kyoto University, my research contributed to the development of new synthetic methodologies and functional organic molecules with applications across various fields.
Taking the helm of the Noyori Laboratory at Nagoya University at the age of 33, I aimed to lead impactful research with a focus on molecular groups that would influence materials science and chemical biology. Pioneering the field of “molecular nanocarbon science,”1 my research introduced novel nanocarbon structures globally, earning recognition such as “The Molecule of the Year” from Chemical & Engineering News (American Chemical Society) several times. Collaborative efforts with companies resulted in the active integration of molecular nanocarbons into materials science, particularly in organic electronics, establishing molecular nanocarbon science as a significant trend in chemistry.
Concurrently with advancing molecular nanocarbon science, I founded the Institute of Transformative Bio-Molecules (ITbM) at Nagoya University,2 operating under the World Premier International Research Centers Initiative (WPI). As the founding director, I initiated interdisciplinary research integrating synthetic chemistry, plant science, animal science, and theoretical chemistry. This pioneering approach aimed to tackle new challenges and broaden the scope of research.
In April 2024, I relocated my research base to RIKEN, embarking on groundbreaking research in molecular creation chemistry. The following outlines our main research achievements to date and outlines future research directions.
2. Summary of main research achievements
2-1. Programmed chemical synthesis and catalyst rapid synthesis
During my tenure as an assistant professor at Kyoto University, my research introduced the concept of programmable molecule construction and devised innovative methods, such as the removable directing group strategy, to achieve rapid synthesis of catalysts.3 Focusing primarily on challenging substrates like multisubstituted olefins and extended π-electron systems, which lacked a general, flexible synthesis method, the Itami research group successfully demonstrated diversity-oriented synthesis. outcomes included a library of full-color fluorescent molecules and commercialized anticancer drug candidates, with six compounds developed at Kyoto University reaching commercialization.
2-2. Inception of molecular nanocarbon science
Nanocarbons, represented by fullerenes, carbon nanotubes, and graphene, have revolutionized material science. However, two major unresolved problems exist in this field. Firstly, current synthesis methods do not allow the production of structurally pure carbon nanotubes and graphene. Secondly, numerous nanocarbons with novel structures have only been proposed theoretically. Therefore, the key to advancing materials science lies in the development of synthetic chemistry capable of providing structurally pure or entirely new nanocarbons. Aiming to freely synthesize, utilize, and understand nanocarbons as structurally pure molecules, I pioneered the field of “molecular nanocarbon science” rooted in organic chemistry and synthetic chemistry, exploring the challenges faced by nanocarbon science. My research has provided clear solutions to the “mixture problem” and “unsynthesized problem.”1
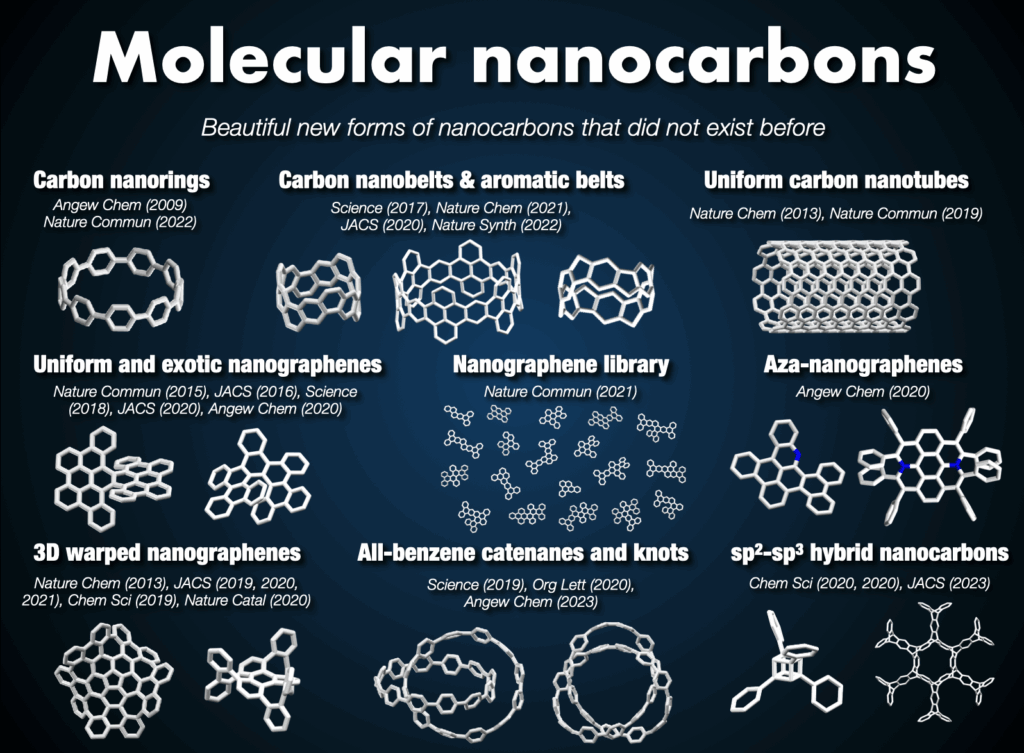
2-3. Synthesis of carbon nanorings and nanobelts
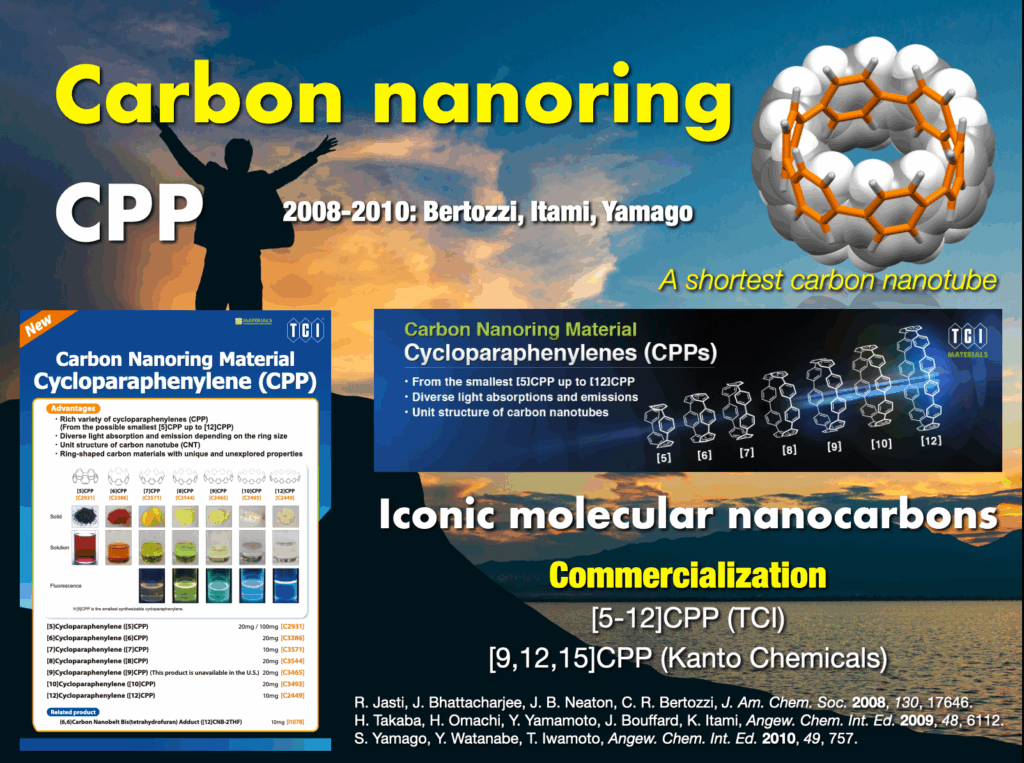
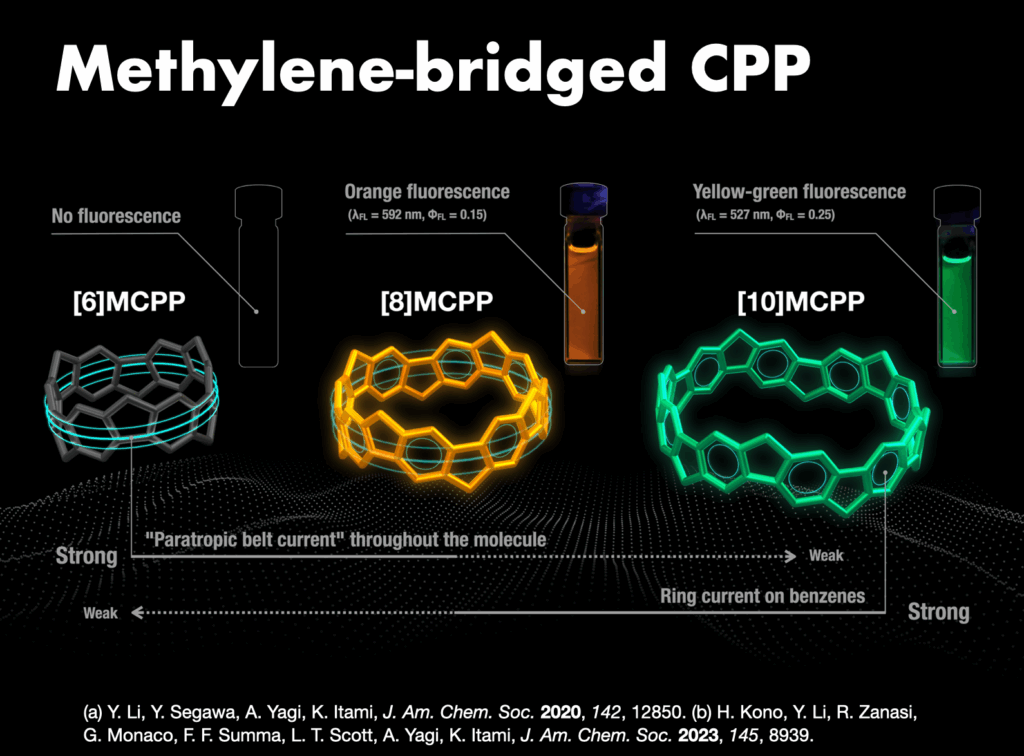
In 2005, I embarked on a groundbreaking quest to synthesize the world’s first carbon nanorings and carbon nanobelts, regarded as structurally pure forms of carbon nanotubes. These targets had long been deemed impregnable.4 By 2009, my research team successfully synthesized carbon nanorings (cycloparaphenylene: CPP),5 a feat also achieved independently by Bertozzi’s group in the United States around the same time.6 CPP has become a subject of global study, with recent advancements in practical applications spanning devices and various fields. The Itami group’s notable achievements include an efficient CPP synthesis method, commercialization of seven CPP compounds,7 and the synthesis of diverse derivatives like perfluorinated CPPs8 and methylene-bridged CPPs.9,10 Our work extends to the discovery of unique optoelectronic properties, advancements in supramolecular chemistry, development of host-guest chemistry,11 stimuli-responsive materials in bioimaging applications,12 and the use of CPP as a template for carbon nanotube synthesis.13
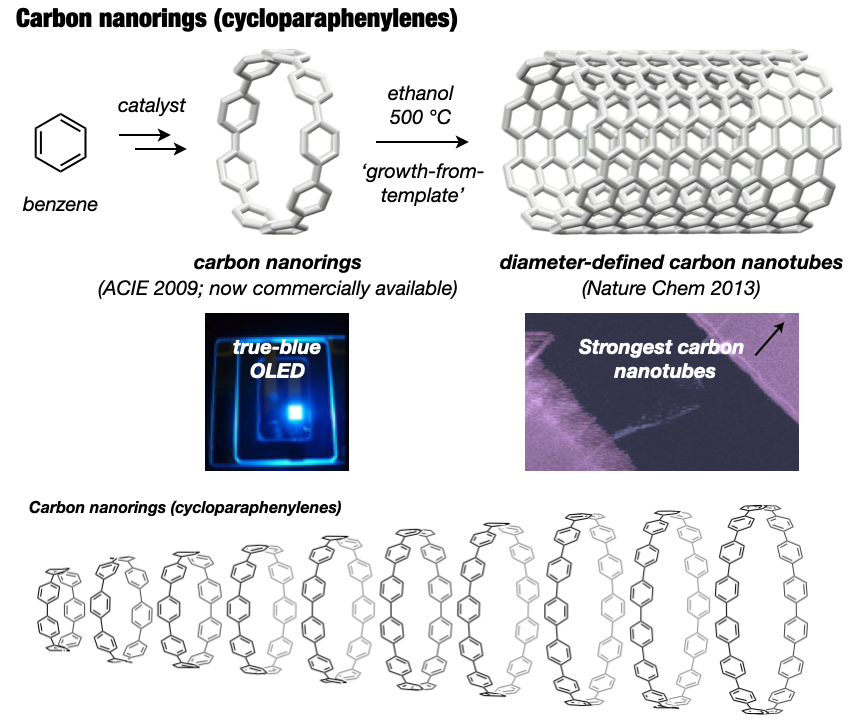
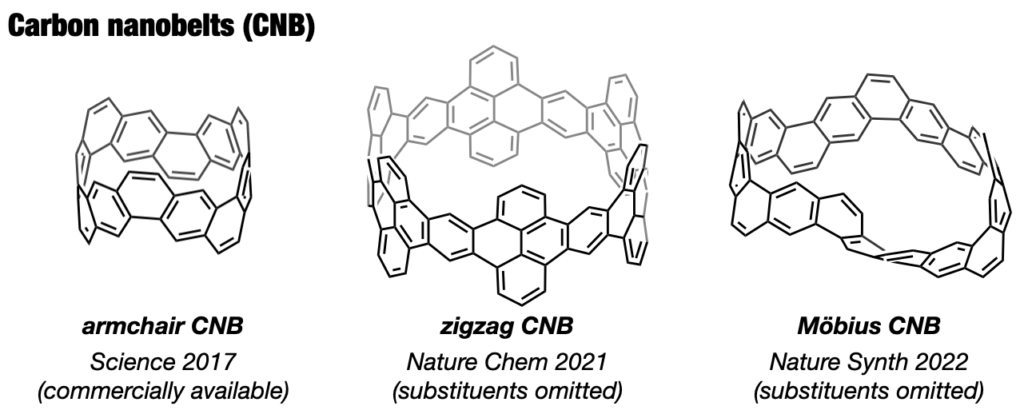
In 2017, our group achieved the synthesis of a thick and fully fused carbon nanobelt (CNB);14 essentially an ultrashort carbon nanotube. Despite its deceptive simplicity, synthesizing the nanobelt presented extreme difficulty due to the high strain on the benzene rings required for its formation. Despite their beautiful structures and potential applications in semiconductors and photonics, their synthesis has alluded scientists for more than 60 years. After 12 years of dedicated efforts, the team not only enhanced synthesis efficiency,15 contributing to CNB commercialization16 but also achieved more challenging CNB variants with zigzag edges17 and a Möbius topology.18 These sought-after CNBs are now employed as functional nanomaterials across diverse fields, marking a significant trend in chemistry.


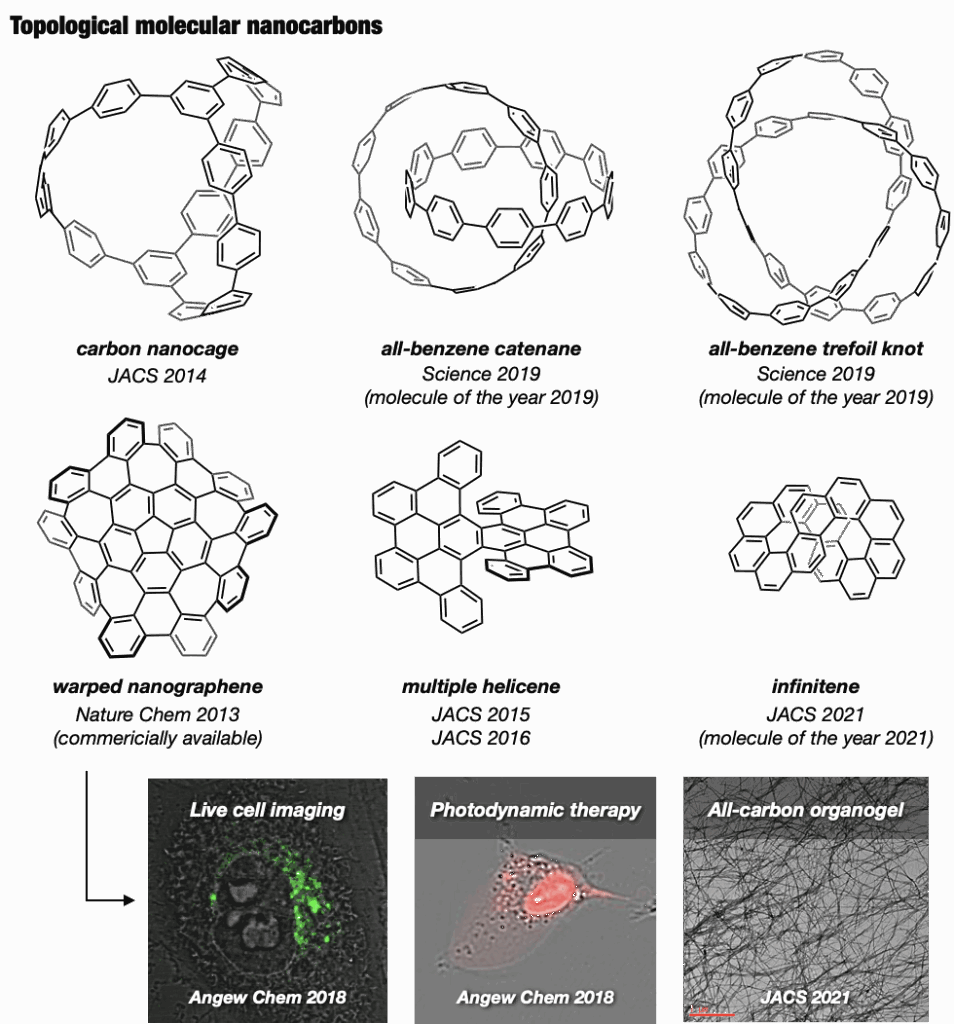
2-4. Synthesis of topologically unique molecular nanocarbons
In the Itami laboratory, we also innovated an expandable collection of topologically unique molecular nanocarbons, with the synthesis of the first carbon accomplished in 2013. Of particular interest are nanocages,19 mechanically interlocked molecules like catenanes, rotaxanes, and molecular knots, prized for their appealing structures and potential applications in molecular machines. In 2019, our team successfully synthesized an all-benzene catenane and trefoil knot, providing fully characterized structures.20
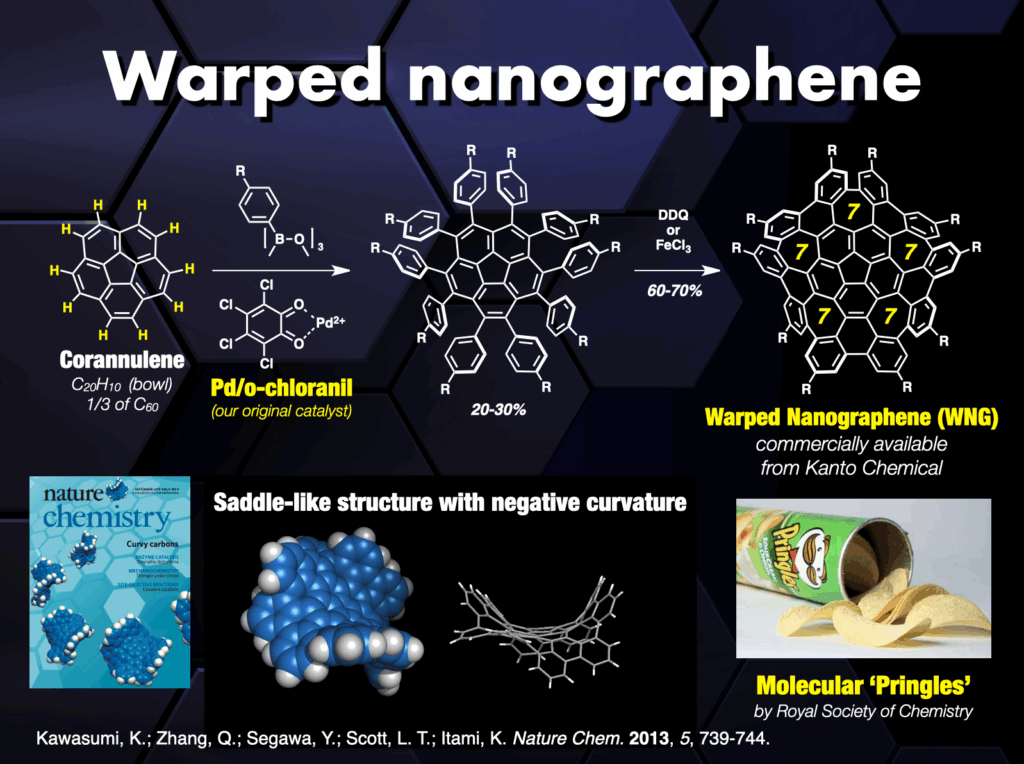
Beyond these achievements, three-dimensional curved nanocarbons remained unexplored until 2013, when, in collaboration with Larry Scott, we synthesized a novel warped nanographene (WNG) featuring both positive and negative curvatures on its π-surface.21 WNGs distinguish themselves from other nanocarbons by their negatively curved geometry imparting a flexible configuration in the solution. Commercially available WNGs are now integral components in various industries,22 particularly in optoelectronic devices.
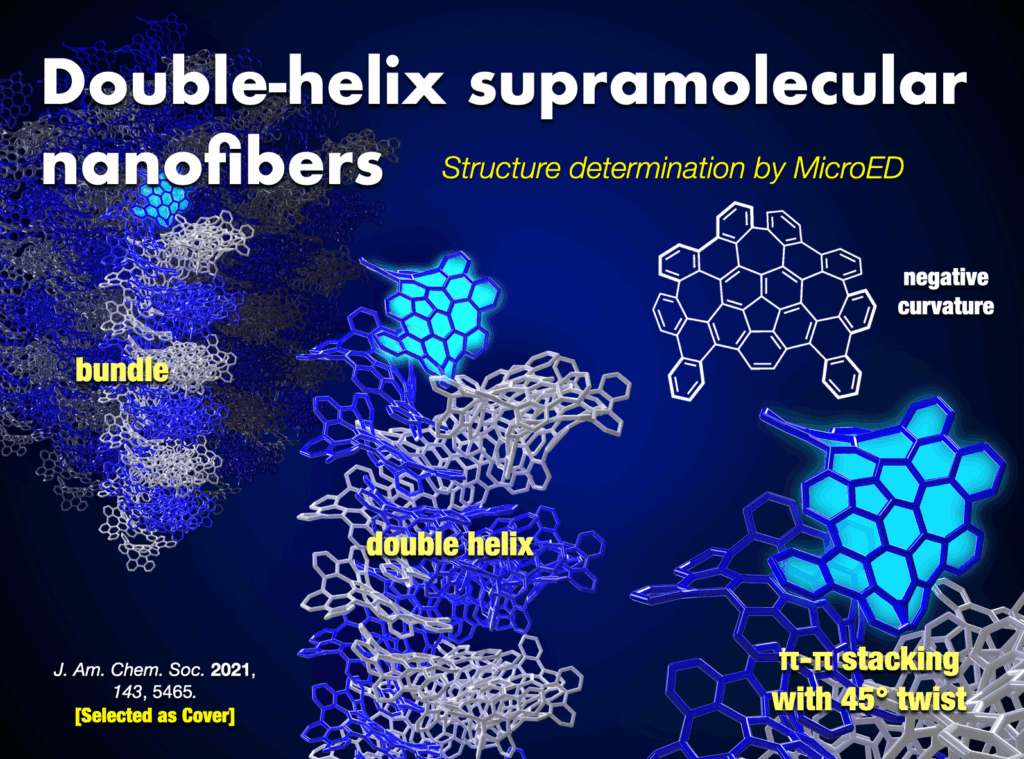
Our team also developed a water-soluble WNG with long-lived green-yellow fluorescence, excellent photostability, and remarkably low cytotoxicity to cells.21 This water-soluble WNG, when introduced into HeLa cells, induced cell death by light irradiation, showcasing its potential in photodynamic therapy. Furthermore, WNGs exhibited the ability for one-dimensional self-assembly, resulting in the creation of the first all-carbon organogels.21
At Itami’s group, we further expanded our portfolio by synthesizing helically twisted nanocarbons, including multiple helicenes23 and the infinity-shaped figure-of-eight nanocarbons (infinitene), which was recognized as the molecule of the year in 2021.24 This diverse collection of topologically unique molecular nanocarbons is poised to open new avenues in organic chemical synthesis, material design, and applied technology.
During this period, the Itami group pioneered the fusion of nanocarbon synthesis chemistry, condensed matter physics,25 and supramolecular/carbon materials chemistry,26 through the JST-ERATO project. Collaborative research with companies has facilitated the incorporation of molecular nanocarbons into materials science applications, particularly in the realm of organic electronics.

2-5. New reactions and catalysts for intuitive molecular assembling and editing
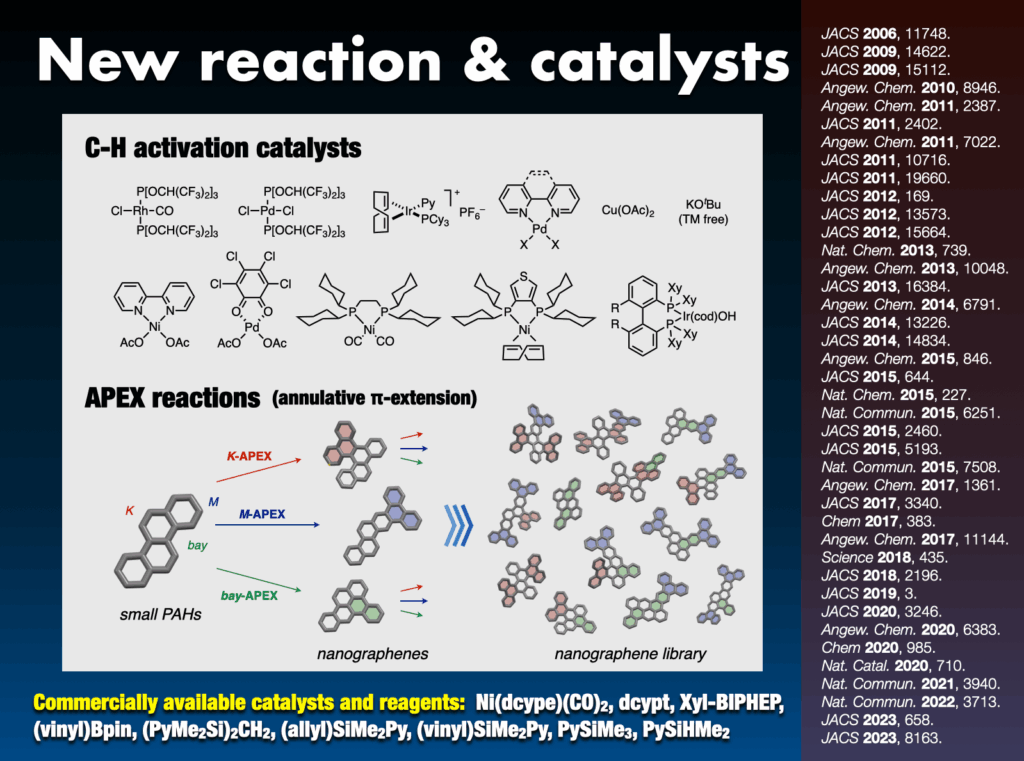
Synthetic chemistry serves as the lifeblood for tailoring molecules with diverse structures. My group has pioneered the development of numerous novel chemical reactions that activate and connect molecules in an “intuitive” and “direct” manner, transcending the limitations found in traditional textbooks. Notably, the group has successfully accomplished direct carbon–hydrogen (C–H) bond activation in aromatic compounds, abundant in nature, enabling the rapid synthesis of arene-assembled molecules such as molecular nanocarbons. Their arsenal includes approximately 20 unique C–H transformation catalysts,27 widely adopted by researchers worldwide. Additionally, nine commercially available catalysts, ligands, and reagents have been employed in groundbreaking work.
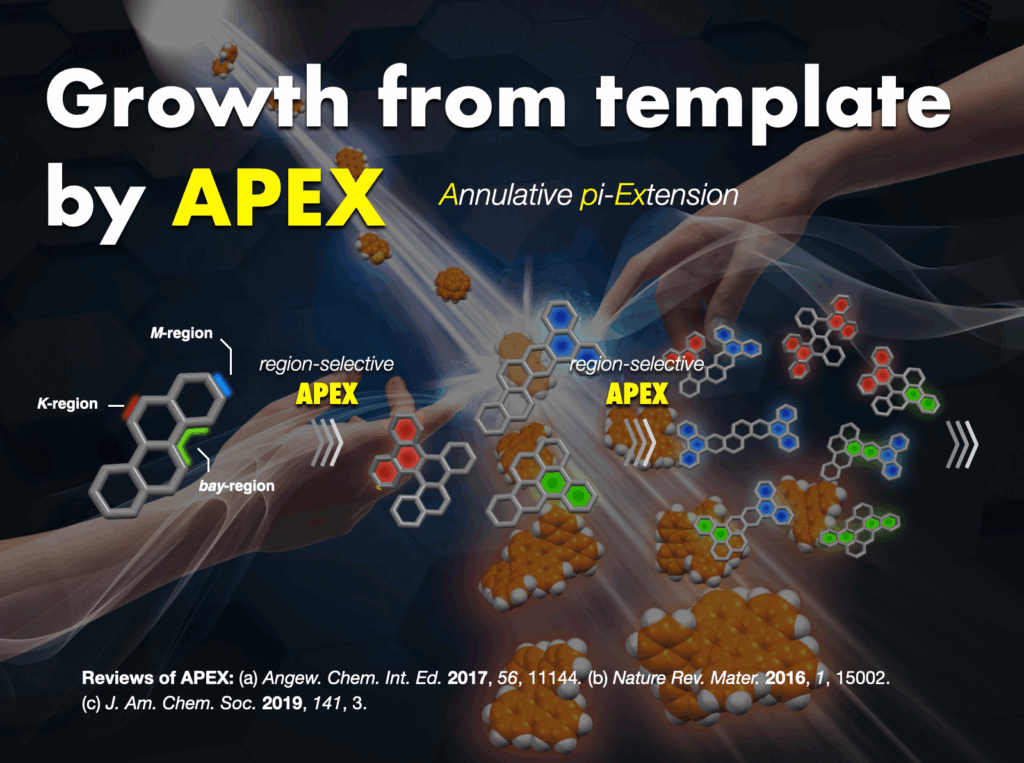
Furthermore, I proposed and successfully developed the annulative π-extension (APEX) reaction28 as an innovative synthetic methodology. This technique facilitates the rapid synthesis and direct conversion of nanographene, leading to the creation of various functional nanocarbon molecules. The APEX reaction represents a significant stride in the field, providing a versatile approach for synthesizing complex nanocarbon structures with broad applications.

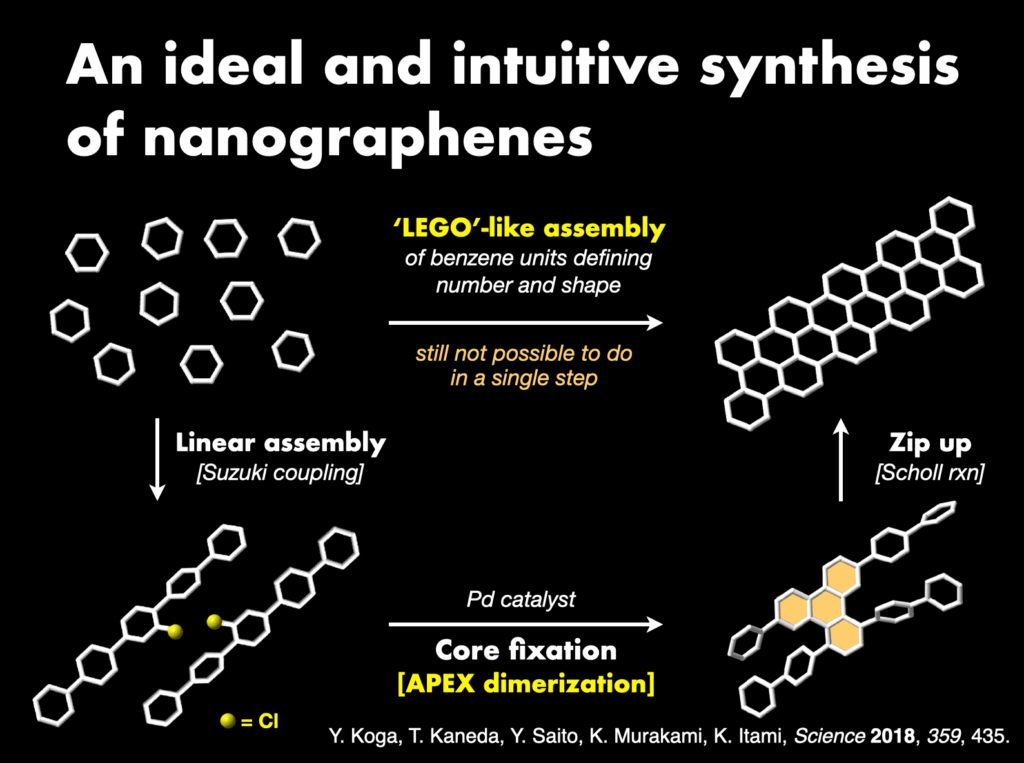
2-6. The launch of ITbM and collaboration with plant and animal biologists, and theoretical chemists
In 2012, I established the Institute of Transformative Bio-Molecules (ITbM) at Nagoya University. Here, the Mix-Lab concept was introduced, fostering an environment where researchers from diverse fields could collaborate freely, transcending traditional boundaries. The overarching goal was to pioneer new fields of interdisciplinary research guided by the mantra “changing the world with molecules.”
As the founding director of ITbM, I have played a pivotal role in leading cutting-edge research. In my capacity as a principal investigator, I have actively contributed to the development of several groundbreaking molecules. These include inhibiting molecules for the parasitic plant Striga,29 stomatal controlling molecules30modulating
Over the years, ITbM has evolved into a world-leading international research institute, seamlessly integrating various fields of study. The collaborative and interdisciplinary nature of ITbM has positioned it as a hub for innovative research, marked by the synthesis of transformative molecules and their applications across diverse scientific domains.35


3. Future research directions
Synthetic chemistry stands out as the most creative discipline, offering boundless opportunities to create value. The imaginative process of molecular design, coupled with the creativity of molecular assembly and editing technology, ensures an endless array of targets. A molecular model, combined with powerful chemical reactions, becomes the gateway to unlocking infinite possibilities in terms of physical and biological properties and functions. Notably, the study of fullerenes serves as a representative example, showcasing the unforeseen properties and functions hidden within. However, new forms of carbon often possess undiscovered or unprecedented properties, unknown at the time of their discovery.
In the realm of molecules, history attests that aesthetically pleasing structures often coincide with remarkable functionality. Molecules possess the transformative power to change the world. Reflecting on the history of science, breakthroughs related to the creation and discovery of new molecules, as well as the exploration of unprecedented physical and biological properties, have led to nonlinear advancements. Moreover, the innovative connection of molecules and fields seemingly unrelated in common sense can lead to the creation of new value, echoing Steve Jobs’ famous quote about “connecting the dots.”
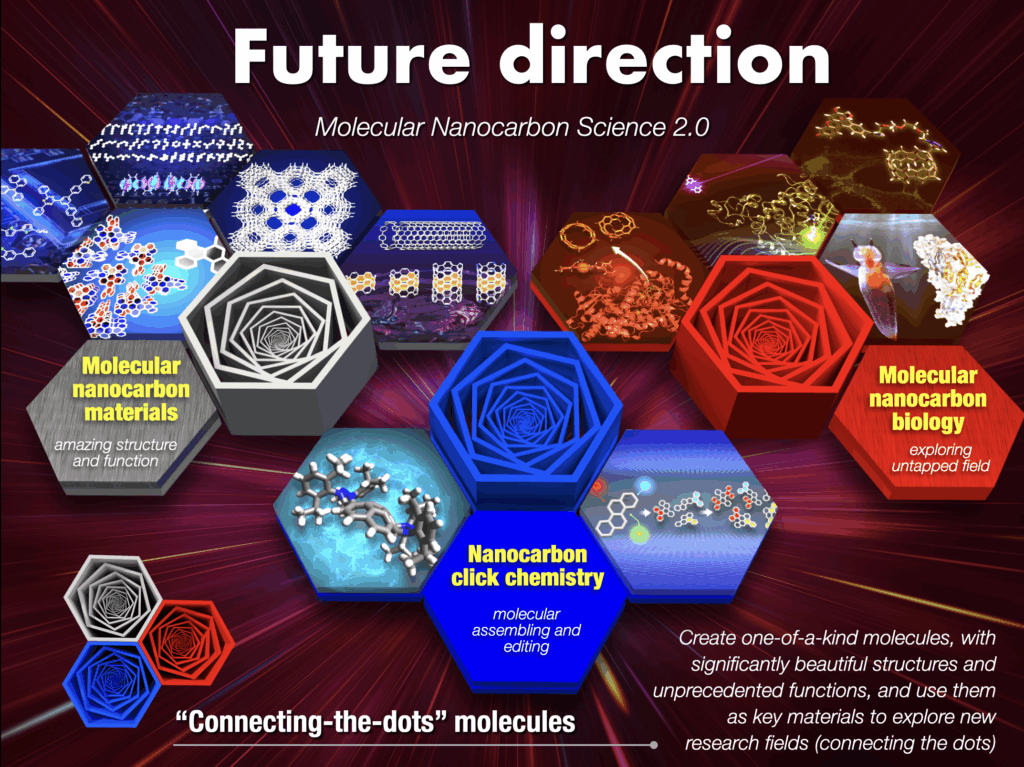
Our approach involves the simultaneous creation of molecules that embody these innovation factors. We embark on an exploration of uncharted territories, aspiring to craft unique molecules that blend structural beauty with unparalleled functionality, thereby creating new materials. Additionally, we aim to activate our molecules in the unexplored boundaries between fields that have never coexisted before, delving into new frontiers. Our commitment to molecule creation chemistry is grounded in three key pillars: nanocarbon click chemistry for “molecular synthesis and editing,” molecular nanocarbon materials science focusing on “extraordinary structure and function,” and molecular nanocarbon biology dedicated to “exploring uncharted territories.” Through this multidimensional approach, we aim to drive innovation and shape the future landscape of science and technology.
3-1. Nanocarbon click chemistry
Fused aromatic compounds, like nanographene, constitute a vital class of compounds extensively utilized in electronic materials, including organic thin-film transistors, solar cells, and organic electroluminescence devices. These compounds are increasingly recognized as “molecular nanocarbons,” representing a crucial avenue for harnessing nanocarbons as “molecules” in the future. However, the synthesis of molecular nanocarbons encounters challenges such as “multi-step-process problems” and “unsynthesized problems.” Our team has pioneered novel synthetic methodologies, such as the APEX reaction, revolutionizing the synthesis of molecular nanocarbons and fused-ring aromatic compounds.
In the pursuit of “molecular nanocarbon biology,” a key focus of our future research, synthesis difficulties pose a bottleneck in the biological applications of these compelling compounds. Hence, developing a more robust synthetic method is essential. We are set to develop the “nanocarbon click reaction” as a new concept, expected to significantly impact not only molecular nanocarbon biology research but also future research in molecular nanocarbon materials science and a wide array of fields.
Molecular nanocarbons and “functional” molecules, encompassing organic electronic materials, plastics, medicines, agrochemicals, proteins, and amino acids, predominantly feature benzene rings. These rings, serving as a ubiquitous skeletal structure, play diverse functional roles. However, benzene rings, known for their stability and low reactivity, are typically used as the backbone of functional molecules. The transformative potential lies in the ability to functionalize benzene rings under “selective” and “mild” conditions, akin to click reactions. Our endeavors have resulted in advancements like the direct conversion of benzene rings into molecular nanocarbons and bioactive molecules (catalytic C–H activation) and the APEX reactions. However, their reactivity and selectivity remain insufficient, necessitating high temperatures and catalysts. Our ultimate goal is to apply these reactions to complex systems, aiming for direct intracellular conversion.
To address these challenges, we are committed to establishing nanocarbon click chemistry, targeting nanocarbon structures and benzene rings. We aim to elevate the reaction power to a level comparable to the click reaction that earned the Nobel Prize in Chemistry in 2022. Nanocarbon click chemistry is pivotal, unlocking possibilities for the development of both molecular nanocarbon materials science and molecular nanocarbon biology.
3-2. Molecular nanocarbon materials science
While we have successfully synthesized a variety of molecular nanocarbons and investigated their functionalities, our current objective is to design unique molecules that outperform conventional ones in both structure and function. Our ultimate ambition is to craft a molecule surpassing the capabilities of even fullerenes.
3-2-1. Synthesis of ultimate molecular nanocarbons
We have successfully synthesized nanocarbons with diverse curved and flat structures. At RIKEN, our current emphasis is on synthesizing three molecules that can be regarded as ultimate structures. Looking ahead, we plan to introduce specific molecular structures in our ongoing research.
3-2-2. All-carbon electronic materials
In the field of organic electronics, materials that exhibit fundamental physical properties such as hole transport, electron transport, semiconductor conductivity, and luminescence are indispensable. The field has witnessed remarkable progress through the discovery and advancement of superior organic materials. Triarylamines with nitrogen atoms dominate hole-transport materials, while electron-transport materials are led by electron-deficient heterocycles and fullerene derivatives. Polythiophenes and oligothiophenes are prevalent in the domain of organic semiconductors. The hallmark of these molecular groups lies in the incorporation of heteroatoms to attain desired physical properties.
However, we aim to challenge this conventional wisdom. Our goal is to disrupt this “common sense” by introducing all-carbon electronic materials composed solely of carbon and hydrogen atoms. We aspire to establish these materials as the new standard, ushering in a nonlinear breakthrough in the field of organic electronics.
3-2-3. Structure-property mapping of molecular nanocarbons
While we possess strengths in synthetic chemistry, the development of the aforementioned materials poses a significant challenge, requiring extensive time and manpower through the conventional “molecular design → synthesis → physical property evaluation” approach. This challenge is attributed to the extremely large diversity of nanographene structures. Even when restricting our focus to nanographene, a polycyclic aromatic hydrocarbon with 10 or fewer six-membered rings, the theoretically feasible structures amount to an overwhelming 20,620. Compounding the complexity, many of these structurally simple molecules have never been synthesized.
To address this complexity, we employ a multidisciplinary approach, integrating quantum chemical calculations, machine learning, AI, and robotic synthesis to construct structure-property maps of molecular nanocarbons. We aim to expedite the achievement of our goals using cutting-edge nanocarbon synthesis technology by narrowing down the group of candidate molecules that exhibit the desired physical properties.
We envision that the structure-property maps generated for nanographenes and molecular nanocarbons will serve as a kind of “periodic table,” offering researchers worldwide a valuable resource for their endeavors in chemistry. These maps are poised to become a significant asset, contributing to the advancement of research and innovation in the field.
3-3. Molecular nanocarbon biology
Molecular nanocarbons possess distinctive attributes not present in traditional bio-functional molecules, including a broad 2D π-plane, flexible construction of 2D and 3D structures, as well as photo/electronic responsiveness and bioorthogonality. By leveraging these unique features, we are confident in our ability to create molecular nanocarbons that drive bioinnovation through a purposeful molecular design.
We believe that the introduction of the concept of “molecular nanocarbon biology” will enhance the value and creativity of nanocarbons. This concept is poised to contribute to the ongoing social impact of nanocarbons within the field of materials science.
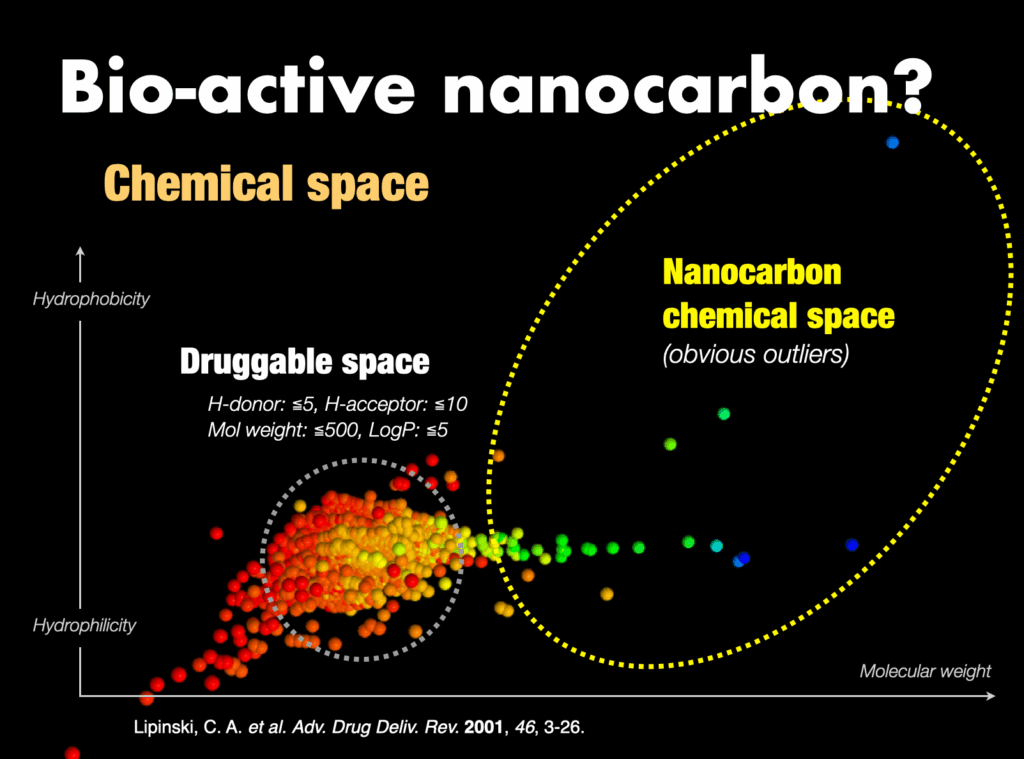

3-3-1. Bioactive molecular nanocarbons
Traditionally, drug discovery has relied on empirical rules like Lipinski’s rule of five to define drug-likeness. However, the chemical space of nanocarbons significantly deviates from established medicinal criteria, leading to skepticism about their, biological activity. Despite this, there has been a lack of thorough verification. Recently, our discovery of several bioactive nanocarbon molecules challenges these perceptions and is poised to usher in guiding principles for the development of bioactive compounds.
Pharmaceutical companies, which have extensively explored drug candidates within the chemical space defined by Lipinski’s rule of five, face challenges in discovering new drugs globally. Molecular nanocarbons, serving as outliers, present the potential for groundbreaking discoveries. The success of our recent research on bioactive nanocarbon molecules represents a significant expansion of the conventional chemical space. This breakthrough is anticipated to establish a new paradigm in drug discovery research, introducing novel exploration areas and revitalizing the pursuit of new therapeutic agents.
3-3-2. Controlling protein-protein interactions by molecular nanocarbons
We are inclined to explore the control of protein-protein interactions (PPIs), as a distinctive avenue for molecular nanocarbons, given their central role in the development of costly antibody drugs. PPIs play crucial roles in various biological processes, including cell proliferation, differentiation, and signal transduction, making them noteworthy targets for novel drug discovery. Although achieving this with conventional small organic molecules is challenging, we believe that the wide π-plane and unique physical properties of molecular nanocarbons offer precise control over PPIs.
Through preliminary screening utilizing our nanocarbon library, we have recently identified a molecular nanocarbon with the capability to regulate PPI. Moving forward, in tandem with optimizing molecular structures and delving into structural biology research, we plan to undertake drug discovery verification, laying a solid foundation for therapeutic drug development. We believe that molecular nanocarbons possess the potential to establish a new standard for PPI-controlling molecules. This is attributed to their high hydrophobicity, abundant aromatic rings, and susceptibility to π–π interactions.
3-3-3. Delivery of proteins and nucleic acids by molecular nanocarbons
Biopolymers like proteins and nucleic acids play crucial roles in basic research and are increasingly gaining prominence as pharmaceuticals (such as antibody drugs and nucleic acid drugs) and tools for genome editing. The direct introduction of proteins is particularly advantageous, as it circumvents the need for transcription, translation, or post-translational modifications associated with protein expression using nucleic acids. This approach is especially ideal for producing induced pluripotent stem (iPS) cells, where precise timing of functional expression is crucial for inducing cell differentiation.
However, biopolymers generally face challenges due to their limited membrane permeability caused by their size and surface charge. Recently, we have designed and synthesized a molecular nanocarbon that demonstrates the ability to transport nucleic acids within mammalian cells. Furthermore, we have identified a nanocarbon molecule capable of transporting proteins, which poses a more significant challenge than nucleic acids. Looking ahead, our goal is to pioneer a new class of standard materials for the transport of proteins and nucleic acids, addressing both fundamental and practical needs in this evolving field.
3-3-4. Nanocarbon peptides
We plan to innovate by integrating nanocarbons with appealing physical properties into widely used peptides in biology and materials science, giving rise to a novel class of materials termed “nanocarbon peptides.” Our exploration will span the realms of chemistry, biology, and materials science. By combining the high bioorthogonality of nanocarbons with the excellent biocompatibility of peptides, these materials can serve as biocompatible substances unaffected by the in vivo environment, particularly valuable in biological applications.
Moreover, incorporating the distinctive π-plane characteristic of nanocarbons into peptides that contribute to aggregate structures opens up possibilities for their use as supramolecular materials with unique assembly modes in the materials field. Nanocarbon peptides represent a pioneering category of “connecting-the-dots” hybrid molecules, enabling molecular nanocarbons to function in a dimension beyond their traditional scope, presenting infinite possibilities.
In our ongoing research, we also plan to explore additional themes, and details about these themes will be introduced later.
4. Our commercially available molecules (22 compounds)
[15]Cycloparaphenylene, [15]CPP (Kanto Chemical)
[12]Cycloparaphenylene, [12]CPP (TCI, Kanto Chemical)
[11]Cycloparaphenylene, [11]CPP (TCI)
[10]Cycloparaphenylene, [10]CPP (TCI)
[9]Cycloparaphenylene, [9]CPP (TCI, Kanto Chemical)
[8]Cycloparaphenylene, [8]CPP (TCI)
[7]Cycloparaphenylene, [7]CPP (TCI)
Methylene-bridged [6]cycloparaphenylene, [6]MCPP (TCI)
(6,6)Carbon nanobelt bis(tetrahydrofuran) adduct, (6,6)CNB (TCI)
Warped nanographene, WNG (Kanto Chemical)
[1,2-Bis(dicyclohexylphosphino)ethane]dicarbonylnickel(0), Ni(dcype)(CO)2 (Kanto Chemical)
3,4-Bis(dicyclohexylphosphino)thiophene, dcypt (Kanto Chemical)
2,2‘-Bis[bis(3,5-dimethylphenyl)phosphino]-1,1’-biphenyl, Xyl-BIPHEP (TCI)
5-Adamantyl-IAA, super-strong auxin (TCI)
Yoshimulactone Green, YLG (TCI)
AMOR, glyco-enhancer of plant fertilization (TCI)
Vinylboronic acid pinacol ester (TCI, Sigma-Aldrich)
Bis[dimethyl(2-pyridyl)silyl]methane (TCI)
2-(Allyldimethylsilyl)pyridine (Sigma-Aldrich)
2-(Dimethylvinylsilyl)pyridine (TCI, Sigma-Aldrich)
2-(Trimethylsilyl)pyridine (TCI, Sigma-Aldrich)
2-(Dimethylsilyl)pyridine (Sigma-Aldrich)
5. References
-
-
- Accounts and reviews: (a) Y. Segawa et al., “Structurally uniform and atomically precise carbon nanostructures”. Nature Rev. Mat. 2016, 1, 15002. DOI: 1038/natrevmats.2015.2. (b) K. Itami et al., “Molecular nanocarbon science: present and future”. Nano Lett. 2020, 20, 4718-4720. DOI: 10.1021/acs.nanolett.0c02143. (c) I. A. Stepek et al., “New paradigms in molecular nanocarbon science” Tetrahedron 2022, 123, 132907. DOI: 10.1016/j.tet.2022.132907.
- https://www.itbm.nagoya-u.ac.jp/en/
- Accounts: (a) K. Itami et al., “Multisubstituted olefins: Platform synthesis and applications to materials science and pharmaceutical chemistry” Chem. Soc. Jpn. 2006, 79, 811-824. DOI: 10.1246/bcsj.79.811. (b) K. Itami et al., “Platform synthesis: A useful strategy for rapid and systematic generation of molecular diversity” Chem. Eur. J. 2006, 12, 3966-3974. DOI: 10.1002/chem.200500958.
- Accounts and reviews: (a) Y. Li et al., “Chemical synthesis of carbon nanorings and nanobelts” Mater. Res. 2021, 2, 681-691. DOI: 10.1021/accountsmr.1c00105. (b) Y. Segawa et al., “Chemical synthesis of cycloparaphenylenes” Phys. Sci. Rev.2017, 2, 137-226. DOI: 10.1515/psr-2016-0102. (c) Y. Segawa et al., “Design and synthesis of carbon nanotube segments” Angew. Chem. Int. Ed. 2016, 55, 5136-5158. DOI: 10.1002/anie.201508384. (d) H. Omachi et al., “Synthesis of cycloparaphenylenes and related carbon nanorings: A step toward the controlled synthesis of carbon nanotubes” Acc. Chem. Res. 2012, 45, 1378-1389. DOI: 10.1021/ar300055x. (e) D. Imoto et al., “Carbon nanobelts: Brief history and perspective” Precis. Chem. 2023, 1, 516-523. DOI: 10.1021/prechem.3c00083.
- (a) H. Takaba et al., “Selective synthesis of [12]cycloparaphenylene” Chem. Int. Ed. 2009, 48, 6112-6116. DOI: 10.1002/anie.200902617. (b) H. Omachi et al., “A modular and size-selective synthesis of [n]cycloparaphenylenes: A step toward the selective synthesis of [n,n] single-walled carbon nanotubes” Angew. Chem. Int. Ed. 2010, 49, 10202-10205. DOI: 10.1002/anie.201005734.
- Jasti et al., “Synthesis, characterization, and theory of [9]-, [12]-, and [18]cycloparaphenylene: Carbon nanohoop structures” J. Am. Chem. Soc. 2008, 130, 17646-17647. DOI: 10.1021/ja807126u.
- https://www.tcichemicals.com/assets/brochure-pdfs/Brochure_FF081_J.pdf
- Shudo et al., “Perfluorocycloparaphenylenes” Nature Commun. 2022, 13, 3713. DOI: 10.1038/s41467-022-31530-x.
- (a) Y. Li et al., “A nonalternant aromatic belt: Methylene-bridged [6]cycloparaphenylene synthesized from pillar[6]arene” Am. Chem. Soc. 2020, 142, 12850-12856. DOI: 10.1021/jacs.0c06007. (b) H. Kono et al., “Methylene-bridged [6]-, [8]-, and [10]cycloparaphenylenes: Size-dependent properties and paratropic belt currents” J. Am. Chem. Soc. 2023, 145, 8939-8946. DOI: 10.1021/jacs.2c13208.
- https://www.tcichemicals.com/assets/brochure-pdfs/Brochure_FF122_J.pdf
- (a) Y. Nakanishi et al., “Size-selective complexation and extraction of endohedral metallofullerenes with cycloparaphenylene” Chem. Int. Ed. 2014, 53, 3102-3106. DOI: 10.1002/anie.201311268. (b) H. Ueno et al., “Cycloparaphenylene-based ionic donor-acceptor supramolecule: Isolation and characterization of Li+@C60Ì[10]CPP” Angew. Chem. Int. Ed. 2015, 54, 3707-3711. DOI: 10.1002/anie.201500544.
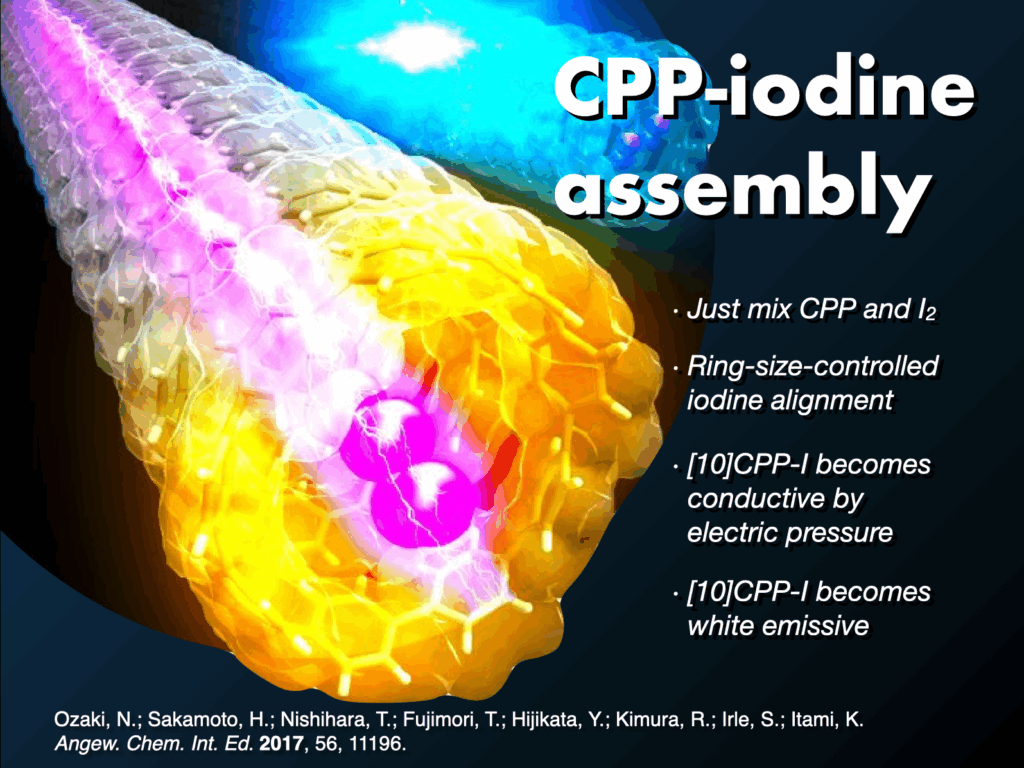
- (a) N. Ozaki et al., “Electrically activated conductivity and white light emission of a hydrocarbon nanoring-iodine assembly”. Chem. Int. Ed. 2017, 56, 11196-11202. DOI: 10.1002/anie.201703648. H. Ishibashi et al., “Noncovalent modification of cycloparaphenylene by catenane formation using an active metal template strategy” Angew. Chem. Int. Ed. 2023, 62, e202310613. DOI: 10.1002/anie.202310613.
- Omachi et al., “Initiation of carbon nanotube growth by well-defined carbon nanorings”. Nature Chem. 2013, 5, 572-576. DOI: 10.1038/nchem.1655.
- Povie et al., “Synthesis of a carbon nanobelt” Science 2017, 356, 172-175. DOI: 10.1126/science.aam8158.
- Povie et al., “Synthesis and size-dependent properties of [12], [16], and [24]carbon nanobelts” J. Am. Chem. Soc. 2018, 140, 10054-10059. DOI: 10.1021/jacs.8b06842.
- https://www.tcichemicals.com/assets/brochure-pdfs/Brochure_FF076_E.pdf
- Y. Cheung et al., “Synthesis of a zigzag carbon nanobelt” Nature Chem. 2021, 13, 255-259. DOI: 10.1038/s41557-020-00627-5.
- Segawa et al., “Synthesis of a Möbius carbon nanobelt”. Nature Synth. 2022, 1, 535-541. DOI: 10.1038/s44160-022-00075-8.
- Matsui et al., “All-benzene carbon nanocages: Size-selective synthesis, photophysical properties, and crystal structure”. J. Am. Chem. Soc. 2014, 136, 16452-16458. DOI: 10.1021/ja509880v.
- Segawa et al., “Topological molecular nanocarbons: all-benzene catenane and trefoil knot” Science 2019, 365, 272-276. DOI: 10.1126/science.aav5021.
- (a) K. Kawasumi et al., “A grossly warped nanographene and the consequences of multiple odd-membered-ring defects” Nature Chem. 2013, 5, 739-744. DOI: 1038/nchem.1704. (b) H.-A. Lin et al., “A water-soluble warped nanographene: Synthesis and applications for photo-induced cell death” Angew. Chem. Int. Ed. 2018, 57, 2874-2878. DOI: 10.1002/anie.201713387. (c) J. I. Urgel et al., “Negatively curved warped nanographene self-assembled on metal surfaces” J. Am. Chem. Soc. 2019, 141, 13158-13164. DOI: 10.1021/jacs.9b05501. (d) S. Matsubara et al., “Creation of negatively curved polyaromatics enabled by annulative coupling that forms an eight-membered ring” Nature Catal.2020, 3, 710-718. DOI: 10.1038/s41929-020-0487-0. (e) K. Kato et al., “Double-helix supramolecular nanofibers assembled from negatively curved nanographenes” J. Am. Chem. Soc. 2021, 143, 5465-5469. DOI: 10.1021/jacs.1c00863.
- https://products.kanto.co.jp/uploads/pj46_m_pdf/111/pdf1.pdf
- (a) Fujikawa et al., “Synthesis, structures, and properties of π-extended double helicene: A combination of planar and nonplanar π-systems” J. Am. Chem. Soc. 2015, 137, 7763-7768. DOI: 10.1021/jacs.5b03118. (b) T. Fujikawa et al., “Synthesis and structural features of quadruple helicenes: Highly distorted π systems enabled by accumulation of helical repulsions” J. Am. Chem. Soc. 2016, 138, 3587-3595. DOI: 10.1021/jacs.6b01303. (c) M. Toya et al., “Expanded [2,1][n]carbohelicenes with 15- and 17-benzene rings” J. Am. Chem. Soc. 2023, accepted.
- Krzeszewski et al., “Infinitene: A helically twisted figure-eight [12]circulene topoisomer” J. Am. Chem. Soc. 2022, 144, 862-871. DOI: 10.1021/jacs.1c10807.
- (a) Hong et al., “Unidirectional molecular assembly alignment on graphene enabled by nanomechanical symmetry breaking” Sci. Rep. 2018, 2333. DOI: 10.1038/s41598-018-20760-z. (b) T. Nishihara et al., “Ultra-narrow-band thermal exciton radiation in intrinsic one-dimensional semiconductors”. Nature Commun. 2018, 9, 3144. DOI: 10.1038/s41467-018-05598-3. (c) A. Takakura et al., “Strength of carbon nanotubes depends on their chemical structures” Nature Commun. 2019, 10, 3040. DOI: 10.1038/s41467-019-10959-7.
- (a) Sakamoto et al., “Cycloparaphenylene as a molecular porous carbon solid with uniform pores exhibiting adsorption-induced softness” Chem. Sci. 2016, 7, 4204-4210. DOI: 10.1039/C6SC00092D. (b) T. Mori et al., “Carbon nanosheets by morphology-retained carbonization of two-dimensional assembled anisotropic carbon nanorings” Angew. Chem. Int. Ed. 2018, 57, 9679-9683. DOI: 10.1002/anie.201803859.
- (a) Yanagisawa et al., “Direct C-H arylation of (hetero)arenes with aryl iodides via rhodium catalysis” J. Am. Chem. Soc. 2006, 128, 11748-11749. DOI: 10.1021/ja064500p. (b) S. Yanagisawa et al., “Programmed synthesis of tetraarylthiophenes through sequential C-H arylation” J. Am. Chem. Soc. 2009, 131, 14622-14623. DOI: 10.1021/ja906215b. (c) K. Mochida et al., “Direct arylation of polycyclic aromatic hydrocarbons through palladium catalysis” J. Am. Chem. Soc. 2011, 133, 10716-10719. DOI: 10.1021/ja202975w. (d) K. Muto et al., “Nickel-catalyzed C-H/C-O coupling of azoles with phenol derivatives” J. Am. Chem. Soc. 2012, 134, 169-172. DOI: 10.1021/ja210249h. (e) Q. Zhang et al., “Palladium-catalyzed C-H activation taken to the limit. Flattening an aromatic bowl by total arylation” J. Am. Chem. Soc. 2012, 134, 15664-15667. DOI: 10.1021/ja306992k. (f) J. Yamaguchi et al., “C-H bond functionalization: Emerging synthetic tools for natural products and pharmaceuticals” Angew. Chem. Int. Ed. 2012, 51, 8960-9009. DOI: 10.1002/anie.201201666. (g) S. Suzuki et al., “Synthesis and characterization of hexaarylbenzenes with five or six different substituents enabled by programmed synthesis” Nature Chem. 2015, 7, 227-233. DOI: 10.1038/nchem.2174. (h) T. Kawakami et al., “Catalytic C-H imidation of aromatic cores of functional molecules: Ligand-accelerated Cu catalysis and application to materials- and biology-oriented aromatics” J. Am. Chem. Soc. 2015, 137, 2460-2463. DOI: 10.1021/ja5130012. (i) Y. Saito et al., “para-C-H borylation of benzene derivatives by a bulky iridium catalyst” J. Am. Chem. Soc. 2015, 137, 5193-5198. DOI: 10.1021/jacs.5b02052. (j) Y. Segawa et al., “Synthesis of extended π-systems through C-H activation” Angew. Chem. Int. Ed. 2015, 54, 66-81. DOI: 10.1002/anie.201403729. (k) K. Muto et al., “Decarbonylative organoboron cross-coupling of esters by nickel catalysis” Nature Commun. 2015, 6, 7508. DOI: 10.1038/ncomms8508. (l) M. Shibata et al., “C-H arylation of phenanthrene with trimethylphenylsilane by Pd/o-chloranil catalysis: Computational studies on the mechanism, regioselectivity, and role of o-chloranil” J. Am. Chem. Soc. 2018, 140, 2196-2205. DOI: 10.1021/jacs.7b11260. (m) Y. Saito et al., “Selective transformation of strychnine and 1,2-disubstituted benzenes by C-H borylation” Chem 2020, 6, 985-993. DOI: 10.1016/j.chempr.2020.02.004. (n) K. Fujishiro et al., “Lithium-mediated mechanochemical cyclodehydrogenation” J. Am. Chem. Soc. 2023, 145, 8163-8175. DOI: 10.1021/jacs.3c01185. (p) K. E. Yamada et al., Angew. Chem. Int. Ed. 2023, 62, e202311770. DOI: 10.1002/anie.202311770.
- (a) Ozaki et al., “One-shot K-region-selective annulative π-extension for nanographene synthesis and functionalization” Nature Commun. 2015, 6, 6251. DOI: 10.1038/ncomms7251. (b) H. Ito et al., “Annulative π-extension (APEX): Rapid access to fused aromatics, heteroaromatics, and nanographenes” Angew. Chem. Int. Ed. 2017, 56, 11144-11164. DOI: 10.1002/anie.201701058. (c) W. Matsuoka et al., “Rapid access to nanographenes and fused heteroaromatics by palladium-catalyzed annulative π-extension reaction of unfunctionalized aromatics with diiodobiaryls” Angew. Chem. Int. Ed.2017, 56, 12224-12228. DOI: 10.1002/anie.201707486. (d) Y. Koga et al., “Synthesis of partially and fully fused polyaromatics by annulative chlorophenylene dimerization” Science 2018, 359, 435-439. DOI: 10.1126/science.aap9801. (e) H. Ito et al., “Polycyclic arene synthesis by annulative π-extension” J. Am. Chem. Soc. 2019, 141, 3-10. DOI: 10.1021/jacs.8b09232. (f) W. Matsuoka et al., “Diversity-oriented synthesis of nanographenes enabled by dearomative annulative π-extension” Nature Commun. 2021, 12, 3940. DOI: 10.1038/s41467-021-24261-y. (g) W. Matsuoka et al., “π-Extended rubrenes via dearomative annulative π-extension reaction” J. Am. Chem. Soc. 2023, 145, 658-666. DOI: 10.1021/jacs.2c11338.
- Tsuchiya et al., “Probing strigolactone receptors in Striga hermonthica with fluorescence” Science 2015, 349, 864-868. DOI: 10.1126/science.aab3831.
- (a) Ziadi et al., “Discovery of synthetic small molecules that enhance the number of stomata: C-H functionalization chemistry for plant biology” Chem. Commun. 2017, 53, 9632-9635. DOI: 10.1039/C7CC04526C. (b) Y. Toda et al., “Identification of stomatal-regulating molecules from de novo arylamine collection through aromatic C-H amination” Sci. Rep. 2022, 12, 949. DOI: 10.1038/s41598-022-04947-z. (c) A. Ueda et al., “Discovery of 2,6-dihalopurines as stomata opening inhibitors: Implication of an LRX-mediated H+-ATPase phosphorylation pathway” ACS Chem. Biol. 2023, 18, 347-355. DOI: 10.1021/acschembio.2c00771. (d) Y. Aihara et al., “Identification and improvement of isothiocyanate-based inhibitors on stomatal opening to act as drought tolerance-conferring agrochemicals” Nature Commun. 2023, 14, 2665. DOI: 10.1038/s41467-023-38102-7.
- (a) Oshima et al., “C-H activation generates period-shortening molecules that target cryptochrome in the mammalian circadian clock” Angew. Chem. Int. Ed. 2015, 54, 7193-7197. DOI: 10.1002/anie.201502942. (b) T. Oshima et al., “Cell-based screen identifies a new potent and highly selective CK2 inhibitor for modulation of circadian rhythms and cancer cell growth” Science Adv. 2019, 5, eau9060. DOI: 10.1126/sciadv.aau9060. (c) T. N. Uehara et al., “Casein kinese 1 family regulates PRR5 and TOC1 in the Arabidopsis circadian clock” Proc. Nat. Acad. Sci. 2019, 116, 11528-11536. DOI: 10.1073/pnas.1903357116. (d) S. Miller et al., “Isoform-selective regulation of mammalian cryptochromes” Nature Chem. Biol. 2020, 16, 676-685. DOI: 10.1038/s41589-020-0505-1. (e) K. Amaike et al., “Small molecules modulating mammalian biological clocks: Exciting new opportunities for synthetic chemistry” Chem 2020, 6, 2186-2198. DOI: 10.1016/j.chempr.2020.08.011. (f) D. Kolarski et al., “Photopharmacological manipulation of mammalian CRY1 for regulation of the circadian clock” J. Am. Chem. Soc. 2021, 143, 2078-2087. DOI: 10.1021/jacs.0c12280. (g) D. Kolarski et al., “Reversible modulation of circadian time with chronophotopharmacology” Nature Commun. 2021, 12, 3164. DOI: 10.1038/s41467-021-23301-x.
- G. Mizukami et al., “The AMOR arabinogalactan sugar chain induces pollen-tube competency to respond to ovular guidance” Curr. Biol. 2016, 26, 1091-1097. DOI: 10.1016/j.cub.2016.02.040.
- B. Frommer, K. Itami, “The prospects of Excellence Initiatives in research: Key ingredients for a successful academic research institute” EMBO Reports 2020, 21, e51398. DOI: 10.15252/embr.202051398.
-


 日本語
日本語
