Making non-emissive [6]cycloparaphenylene fluorescent by simple multiple methyl substitution
Tomoki Kato, Daiki Imoto, Akiko Yagi, Kenichiro Itami
Chem. Sci. 2025, Accepted Manuscript.
URL: https://pubs.rsc.org/en/Content/ArticleLanding/2025/SC/D5SC04694G
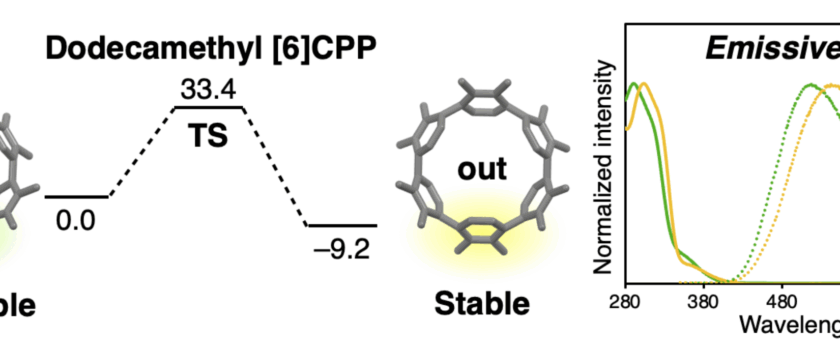
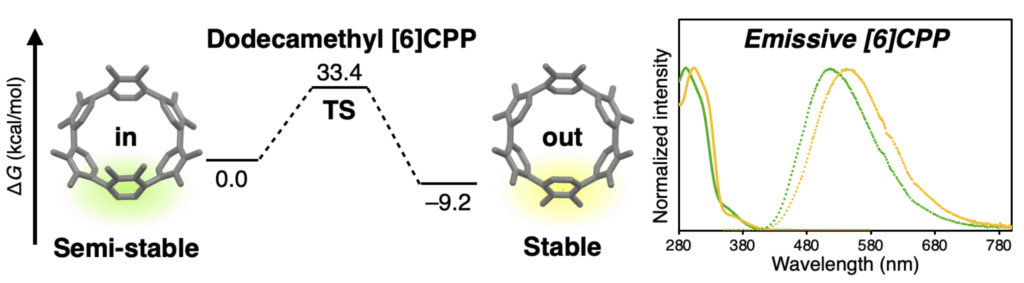
Cycloparaphenylenes (CPPs) have attracted significant interest in various fields such as materials science, chemical biology, and beyond. Quite unexpectedly, we discovered that non-emissive [6]cycloparaphenylene ([6]CPP) can be made fluorescent by multiple additions of simple methyl groups. Dodecamethyl[6]CPP was synthesized and isolated as a pair of isomers (rotamers), both of which fluoresce at 510–540 nm. Multiple methyl substitutions suppress the rotation around the phenylene units, enabling the rotamers to be isolated and their non-radiative interconversion studied. In view of the significance of CPPs as emerging functional molecules in chemistry, materials science, chemical biology, and beyond, we are convinced that this simple yet significant “methyl effect” in CPPs helps clarify the fundamental stereoelectronic effects that act on CPPs, and should facilitate the design and application of CPP-based materials in various fields.


 日本語
日本語