Yuanming Li, Akiko Yagi, Kenichiro Itami*
J. Am. Chem. Soc. 2020, 142, 3246-3253.
DOI: 10.1021/jacs.9b13549
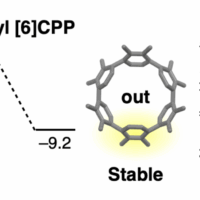
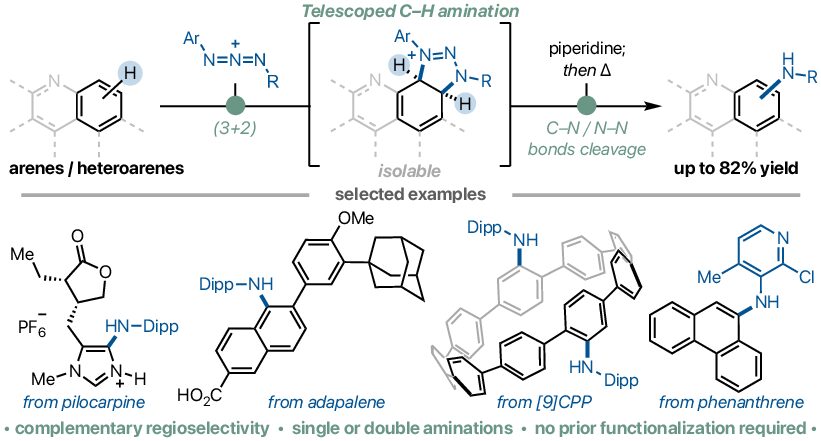
The synthesis, structures, and properties of highly twisted, nonplanar aromatic macrocycles are described. These macrocycles with an approximately 90° twist angle were synthesized by an effective synthetic approach through a quadruple Suzuki–Miyaura coupling of 4,5-bisarylphenanthrene, a novel axially chiral nonplanar building block. By varying the cross-coupling partner as the spacer, a family of twisted macrocycles was synthesized, allowing for a systematic study of the effect of the spacer on macrocycle shape and photophysical properties. For example, a unique macrocyclic aggregation-induced emission (AIE) emitter with double tetraphenylethylene units as the spacers was readily synthesized. Furthermore, attributed to its conformationally restricted twisted structure, a 3,6-disubstituted-1,8-naphthalimide-incorporated macrocycle showed remarkable solvatofluorochromism with high fluorescence quantum yields. The excellent conformational stability of these macrocycles further enabled complete enantiomeric resolution and characterization. The racemization barrier of macrocycle was determined experimentally and supported by DFT calculations.


 English
English