Shin Miyamura, Misaho Araki, Takayoshi Suzuki, Junichiro Yamaguchi, and Kenichiro Itami
Angew. Chem. Int. Ed. 2014, Early view. DOI: 10.1002/anie.201409186
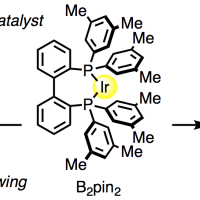
A step-economical and stereodivergent synthesis of privileged 2-arylcyclopropylamines (ACPAs) through a C(sp3)![[BOND]](http://onlinelibrarystatic.wiley.com/undisplayable_characters/00f8ff.gif) H borylation and Suzuki–Miyaura coupling sequence has been developed. The iridium-catalyzed C
H borylation and Suzuki–Miyaura coupling sequence has been developed. The iridium-catalyzed C![[BOND]](http://onlinelibrarystatic.wiley.com/undisplayable_characters/00f8ff.gif) H borylation of N-cyclopropylpivalamide proceeds with cis selectivity. The subsequent B-cyclopropyl Suzuki–Miyaura coupling catalyzed by [PdCl2(dppf)]/Ag2O proceeds with retention of configuration at the carbon center bearing the Bpin group, while epimerization at the nitrogen-bound carbon atoms of both the starting materials and products is observed under the reaction conditions. This epimerization is, however, suppressed in the presence of O2. The present new ACPA synthesis results in not only a significant reduction in the steps required for making ACPA derivatives, but also the ability to access either isomer (cis or trans) by simply changing the atmosphere (N2 or O2) in the coupling stage.
H borylation of N-cyclopropylpivalamide proceeds with cis selectivity. The subsequent B-cyclopropyl Suzuki–Miyaura coupling catalyzed by [PdCl2(dppf)]/Ag2O proceeds with retention of configuration at the carbon center bearing the Bpin group, while epimerization at the nitrogen-bound carbon atoms of both the starting materials and products is observed under the reaction conditions. This epimerization is, however, suppressed in the presence of O2. The present new ACPA synthesis results in not only a significant reduction in the steps required for making ACPA derivatives, but also the ability to access either isomer (cis or trans) by simply changing the atmosphere (N2 or O2) in the coupling stage.


 日本語
日本語